Imagine a world without the vibrant hues of a sunset, the life-giving warmth of the sun, or the shimmering brilliance of a diamond. These seemingly disparate wonders share a common thread: they are all composed of elements, the fundamental building blocks of matter. These elements, arranged in an elegant order known as the periodic table, are the subject of countless scientific inquiries and reveal the underlying beauty and complexity of our universe. Today, we embark on a fascinating journey into the heart of the periodic table, particularly exploring the concept of rounded atomic mass and its implications.

Image: www.pinterest.com
The periodic table, often hailed as the “periodic table of elements,” is a visual masterpiece that unveils the interconnectedness of all matter. It’s more than just a chart – it’s a map, a guide, and a window into the very essence of the world around us. Each element on the table is a unique entity, defined by its atomic number, which signifies the number of protons within its nucleus. It’s this atomic number that determines an element’s identity, much like a fingerprint distinguishes one individual from another. However, there’s another crucial aspect that adds another layer of complexity: atomic mass. Atomic mass, a measure of the total number of protons and neutrons in an atom, is a nuanced value, often expressed as a decimal. While the exact atomic mass is invaluable for precision in scientific calculations, it can sometimes present a barrier to understanding for beginners. This is where the concept of rounded atomic mass comes to the fore, serving as a stepping stone to grasping the fundamentals.
The Rounded Atomic Mass: Simplifying Complexity
The atomic mass of an element is not a fixed value. It varies depending on the abundance of isotopes, which are atoms of the same element with varying numbers of neutrons. While the exact atomic mass, often represented with several decimal places, is essential for precise chemistry, it can be overwhelming for learners. Rounded atomic mass simplifies this complexity. For example, the exact atomic mass of carbon is 12.011 amu (atomic mass units). However, for educational purposes, it’s often rounded to 12 amu. This simplification provides a more manageable starting point, allowing for a deeper understanding of the element’s behavior and its role in various compounds.
The Periodic Table: An Evolutionary Journey
The periodic table we know today wasn’t created overnight; it’s the culmination of centuries of scientific endeavor. From the alchemists of ancient times to the modern-day physicists, the quest to unravel the secrets of matter has been a driving force behind scientific progress.
Dmitri Mendeleev, a Russian chemist, is credited with developing the first recognizable periodic table in 1869. He arranged the elements in order of increasing atomic weight, noticing a pattern in their properties that repeated periodically. This groundbreaking discovery led to the formulation of the periodic law, which states that the properties of elements are periodic functions of their atomic numbers. Mendeleev’s brilliance lay in his insight to leave gaps in his table, predicting the existence of undiscovered elements based on the recurring patterns. These predictions were later validated, further solidifying the table’s significance.
The periodic table has undergone numerous revisions since Mendeleev’s time, incorporating new discoveries and refining our understanding of atomic structure. Today, the table encompasses 118 known elements, each with its own unique characteristics. These elements are categorized into groups (columns) and periods (rows), based on their electron configurations and chemical properties.
Decoding the Periodic Table
The periodic table is a treasure trove of information, presenting a systematic organization of the elements and their properties. Here’s a breakdown of its important components:
- Groups (Columns): Elements within the same group share similar chemical properties due to having the same number of valence electrons, which are the electrons involved in chemical bonding. Group 1, for instance, contains the alkali metals – lithium, sodium, potassium, rubidium, cesium, and francium. These elements are highly reactive and readily form cations (positively charged ions) by losing one electron.
- Periods (Rows): Elements within the same period have the same number of electron shells, which are the regions of space around the nucleus where electrons are found. Elements in the same period exhibit a gradual change in properties, progressing from metallic to non-metallic.
- Metals, Non-metals, and Metalloids: The periodic table is further classified into distinct categories based on the elements’ physical and chemical properties. Most elements are metals, which are typically lustrous, malleable, ductile, and good conductors of heat and electricity. Non-metals, on the other hand, are generally brittle, dull, and poor conductors. Metalloids, also known as semi-metals, possess properties intermediate between those of metals and non-metals, often exhibiting both metallic and non-metallic characteristics.
- Atomic Mass and Isotopes: As we’ve discussed, the atomic mass of an element is a key property. However, it’s important to note that the atomic mass listed on the periodic table is the average atomic mass, taking into account the relative abundance of isotopes in nature. Isotopes are variants of the same element with differing numbers of neutrons. For example, carbon has two common isotopes: carbon-12 (6 protons and 6 neutrons) and carbon-13 (6 protons and 7 neutrons). The average atomic mass of carbon reflects the weighted average of these isotopes’ masses, resulting in the value 12.011 amu.
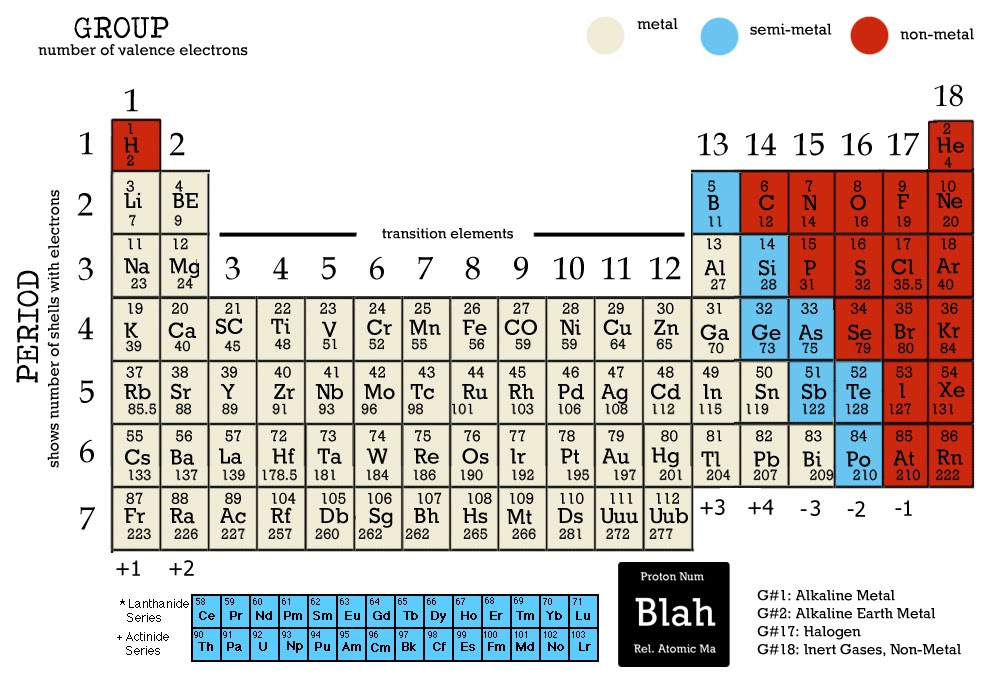
Image: mungfali.com
The Periodic Table: An Empowering Tool
The periodic table is not merely a static chart; it’s a dynamic and ever-evolving tool that empowers us to explore the world with newfound understanding. It offers a framework for comprehending the diversity of elements, predicting their chemical behavior, and unlocking their potential applications. Here are a few ways in which the periodic table empowers us:
- Understanding Chemistry: The periodic table is the backbone of chemistry, providing a fundamental framework for understanding chemical reactions, bonding, and the formation of molecules. By grasping the periodic trends in properties, we can predict how elements will interact with each other and what compounds they are likely to form.
- Developing New Technologies: The periodic table serves as a roadmap for material scientists and engineers in developing innovative materials with specific properties tailored to diverse applications. For instance, silicon, a metalloid element found in Group 14, is crucial to the semiconductor industry, powering countless electronic devices.
- Safeguarding Our Planet: The periodic table plays a vital role in environmental science and sustainability. It helps us understand the environmental impact of various elements and compounds, guiding us towards responsible resource management and pollution control.
Mastering the Periodic Table
Navigating the periodic table might seem daunting at first glance, but with a little practice and patience, it becomes an invaluable tool. Here are some tips for mastering the periodic table and unlocking its potential:
- Start by Familiarizing Yourself with the Basics: Learn the names, symbols, and atomic numbers of common elements.
- Explore the Periodic Trends: Understand the relationships between atomic size, ionization energy, electronegativity, and metallic character.
- Utilize Visual Aids: Create flashcards, posters, or digital resources to reinforce your understanding.
- Engage in Practice Problems: Solve chemistry problems that involve the periodic table to apply your knowledge.
Periodic Table With Rounded Atomic Mass
The Periodic Table: A Journey of Discovery
The periodic table, with its rounded atomic masses, is a testament to the remarkable progress of science and a window into the fundamental building blocks of our universe. It’s a map that guides us, a tool that empowers us, and a source of unending wonder. As we continue to explore the mysteries of matter, the periodic table will undoubtedly remain an essential guide, revealing the beauty and complexity of the world around us. Remember, the journey of discovery never ends; every element on the table has a story to tell, waiting to be unraveled.





