Have you ever wondered how scientists communicate the intricate dance of atoms within molecules? The answer lies within the language of chemical formulas – a shorthand system that unlocks the secrets of the universe’s building blocks. Chemical formulas are more than just a collection of symbols; they are a powerful tool that encapsulates the very essence of matter, revealing its composition, structure, and reactivity.
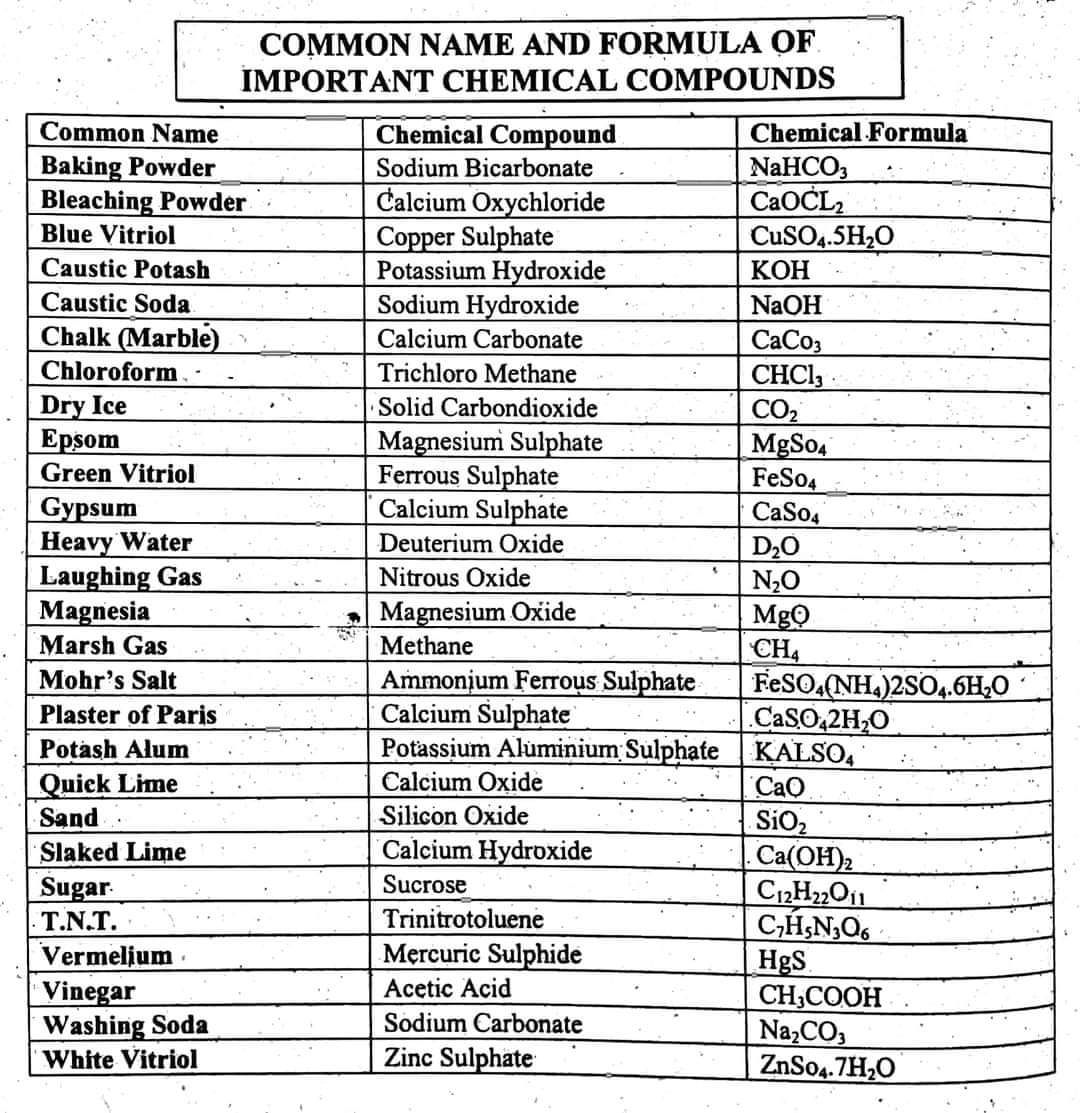
Image: www.chemicalslearning.com
This comprehensive guide delves into the fascinating world of chemical formulas, exploring their history, fundamental principles, and practical applications. Whether you’re a student embarking on your chemical journey or a seasoned scientist seeking to deepen your understanding, this article will provide a thorough exploration of this essential concept.
A Journey Through Time: The Evolution of Chemical Formulas
The journey to understanding chemical formulas is a fascinating tale of scientific ingenuity. Early alchemists, driven by a quest for transmutation, experimented with various substances, often using cryptic symbols to represent their ingredients. However, the modern approach to chemical formulas emerged with the advent of atomic theory.
In the late 18th and early 19th centuries, pioneers like John Dalton and Jöns Jacob Berzelius laid the foundation for modern chemistry. Dalton proposed that elements are composed of indivisible particles called atoms, while Berzelius introduced the system of using letters to represent elements. This system, refined over time, became the basis for the chemical formulas we use today.
Deciphering the Code: Understanding the Basics of Chemical Formulas
Chemical formulas are a symbolic language that concisely represents the composition of molecules. Each formula uses element symbols, derived from the periodic table, along with numerical subscripts to indicate the number of atoms of each element present.
For instance, the formula H2O represents a water molecule, indicating that it consists of two hydrogen (H) atoms and one oxygen (O) atom. Understanding these basic building blocks is crucial to comprehending the complexities of chemical reactions and molecular interactions.
Types of Chemical Formulas: Navigating the Landscape of Representation
The world of chemical formulas is diverse, with several types that provide different levels of information. Let’s explore some common categories:
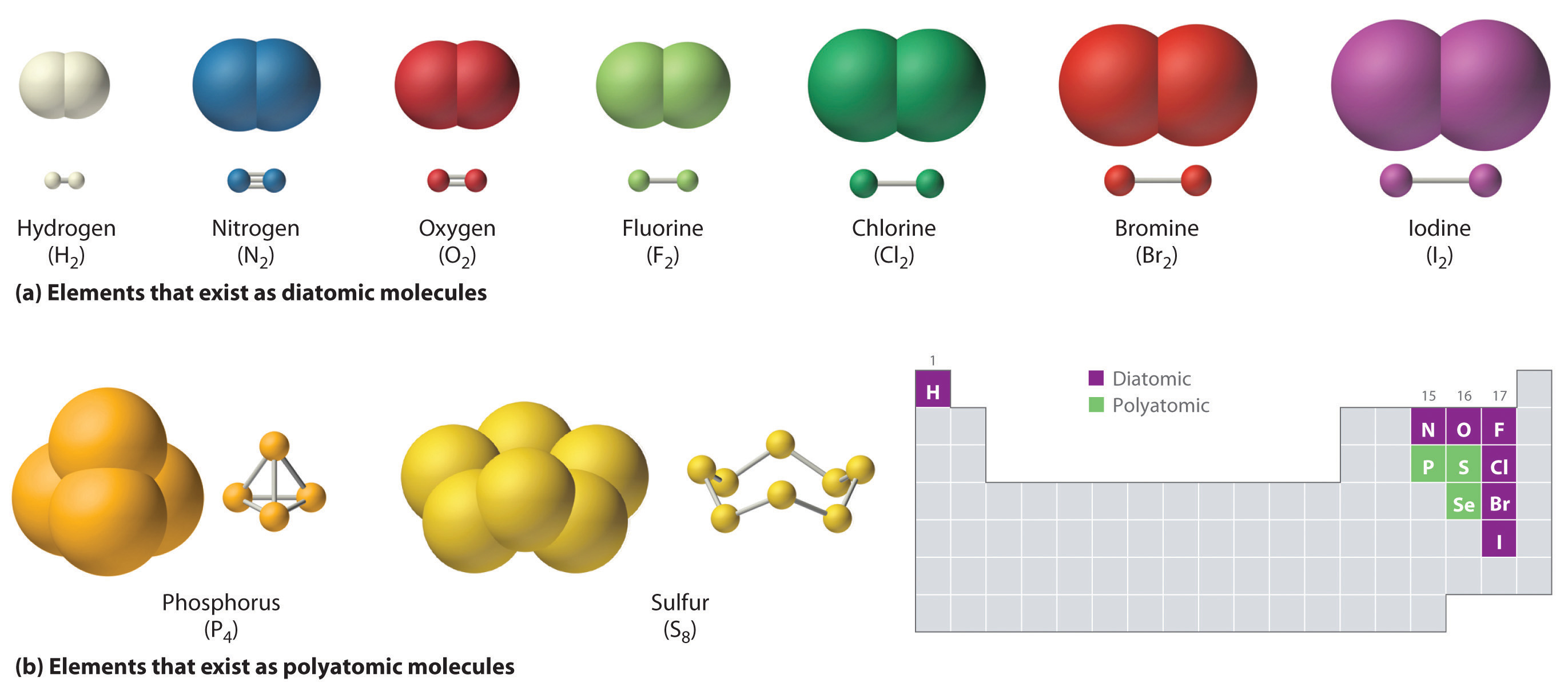
Image: saylordotorg.github.io
Empirical Formulas: The Skeleton Key
Empirical formulas represent the simplest whole-number ratio of atoms in a compound. They reveal the basic composition but don’t necessarily reflect the actual arrangement of atoms. For example, the empirical formula of glucose is CH2O, reflecting the 1:2:1 ratio of carbon, hydrogen, and oxygen atoms.
Molecular Formulas: Unraveling the Complete Picture
Molecular formulas display the exact number of each type of atom present in a molecule. They provide a more complete view compared to empirical formulas. For instance, the molecular formula of glucose is C6H12O6, accurately representing the six carbon, twelve hydrogen, and six oxygen atoms within a glucose molecule.
Structural Formulas: Unveiling the Geometric Dance
Structural formulas go beyond simple composition and depict the arrangement of atoms within a molecule. They often utilize lines to represent bonds between atoms, providing valuable insight into the molecule’s shape and connectivity. For instance, a structural formula of ethanol (C2H5OH) shows the arrangement of carbon, hydrogen, and oxygen atoms, highlighting the presence of a hydroxyl group (-OH).
The Power of Chemical Formulas: Applications Across Disciplines
From understanding fundamental reactions to designing cutting-edge materials, chemical formulas play a pivotal role in various scientific and technological fields:
Chemistry: The Foundation of All
Chemical formulas are the bedrock of chemistry, enabling the prediction and interpretation of chemical reactions. They empower scientists to understand how molecules interact, form new compounds, and transform into different states of matter.
Biochemistry: Deciphering Life’s Complexity
Biochemistry relies heavily on chemical formulas to explore the complex molecules of life. From the DNA that carries our genetic information to the proteins that perform countless cellular functions, chemical formulas provide a framework for understanding the intricate mechanisms of biological systems.
Materials Science: Crafting New Solutions
Materials scientists use chemical formulas to design and synthesize new materials with specific properties. Understanding the composition and structure of materials allows for the creation of advanced polymers, ceramics, and semiconductors that address diverse technological needs.
Pharmaceuticals: Developing Life-Saving Medicines
The development of pharmaceuticals involves a deep understanding of chemical formulas. By manipulating the structure of molecules, scientists can create drugs that target specific biological pathways, offering relief from disease and improving human health.
The Future of Chemical Formulas: Embracing New Horizons
The field of chemical formulas is constantly evolving, with advancements in computational chemistry and experimental techniques pushing the boundaries of our understanding. We are witnessing the development of sophisticated software that can predict and analyze molecular structures with unprecedented accuracy. This progress is fueling the discovery of novel materials and drugs, revolutionizing diverse sectors.
Furthermore, the ongoing development of quantum chemistry offers a deeper understanding of molecular behavior at the atomic level. This allows us to explore complex chemical transformations with greater precision, paving the way for breakthroughs in energy storage, catalysis, and environmental science.
List Of All Formulas In Chemistry
https://youtube.com/watch?v=y33i4JnWMMo
Conclusion: The Enduring Importance of Chemical Formulas
Chemical formulas are more than just symbols on a page; they are a profound language that unlocks the secrets of matter. They provide a framework for understanding the composition, structure, and reactivity of molecules, driving advancement in chemistry, biology, materials science, and countless other fields. As we continue to explore the mysteries of the universe, the importance of chemical formulas will only grow, empowering us to solve challenging problems and create a better future. Explore the vast world of chemical formulas, and you’ll be amazed by the power they hold to illuminate the complexities of our world.





