Have you ever wondered why water is a universal solvent, while oil stubbornly refuses to mix? The answer lies in the fascinating world of molecular polarity, a key concept in chemistry that dictates how molecules interact with each other and their environment. In this guide, we will delve into the intricacies of molecular structure lab reports, specifically focusing on determining the polarity of different molecules. This exploration will not only help you understand the theoretical foundations but also equip you with practical skills for conducting your own laboratory experiments.

Image: www.studocu.com
The concept of molecular polarity is crucial for understanding a vast array of chemical phenomena, impacting everything from drug design and material science to the behavior of biological systems. By understanding the principles of molecular structure and polarity, you gain insights into how molecules interact, their properties, and ultimately, their roles in the world around us. So, let’s embark on this journey of unraveling the secrets of molecular polarity!
Understanding the Basics
Before we venture into the laboratory, let’s establish a firm grasp of the fundamental concepts underlying molecular polarity.
What is Molecular Polarity?
Molecular polarity refers to the distribution of electron density within a molecule, which results in the creation of a permanent electric dipole moment. To simplify, think of a molecule as a tiny magnet with a positive and a negative end. The presence and strength of this dipole moment determine the molecule’s polarity, influencing its behavior in various chemical and physical processes.
Electronegativity: The Driving Force
The concept of electronegativity is essential for understanding molecular polarity. Electronegativity measures an atom’s tendency to attract electrons within a chemical bond. When two atoms with different electronegativities bond, the electrons are unevenly shared, creating a partial positive charge on the less electronegative atom and a partial negative charge on the more electronegative atom.
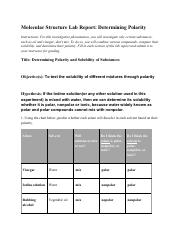
Image: www.coursehero.com
Types of Molecular Polarity
Molecular polarity is broadly categorized into two types:
- Polar Molecules: These molecules exhibit a permanent dipole moment due to the uneven distribution of electron density. For instance, water (H2O) is a polar molecule because oxygen is more electronegative than hydrogen, resulting in a partial negative charge on the oxygen atom and partial positive charges on the hydrogen atoms.
- Nonpolar Molecules: These molecules lack a permanent dipole moment because electrons are shared equally between atoms. For example, methane (CH4) is a nonpolar molecule as the carbon and hydrogen atoms have similar electronegativities, resulting in an even distribution of electrons.
Delving into the Laboratory
Now, let’s dive into the practical aspect of determining molecular polarity through laboratory experiments.
The Molecular Structure Lab Report
A molecular structure lab report is a comprehensive document detailing your experimentation process, observations, and conclusions regarding the polarity of a specific molecule.
Experimental Techniques
Several techniques are employed in molecular structure labs to determine polarity. Some of the most common methods include:
- Solubility Testing: Polar molecules tend to dissolve in polar solvents like water, while nonpolar molecules readily dissolve in nonpolar solvents like oil. Observing the solubility of a substance in different solvents provides valuable insights into its polarity.
- Dipole Moment Measurement: Determining the dipole moment of a molecule using specialized equipment provides a quantitative measure of its polarity.
- Infrared Spectroscopy: This technique analyzes the vibrational frequencies of molecular bonds, providing information about the presence or absence of polar bonds within a molecule.
Analyzing Data and Drawing Conclusions
After conducting your experiments, you will analyze the collected data to draw conclusions about the polarity of your target molecule. This analysis involves comparing your findings to established theories and experimental results, ensuring your conclusions are well-supported and reliable.
Understanding the Implications of Polarity
The concept of molecular polarity extends beyond the confines of the laboratory, impacting various aspects of our world.
Chemical Reactions
Polarity plays a crucial role in chemical reactions. Polar molecules tend to interact readily with other polar molecules, while nonpolar molecules interact more favorably with other nonpolar molecules. This principle governs many chemical reactions, influencing reaction rates and product formation.
Biological Systems
Polar and nonpolar molecules have distinct roles in biological systems. For instance, cell membranes are composed of phospholipid molecules with both polar and nonpolar regions, forming a barrier that regulates the flow of substances into and out of the cell. The interaction of polar molecules like water and nonpolar molecules like fats within the body contributes to the complex processes that keep us alive.
Materials Science
Understanding molecular polarity is essential for developing new materials. The properties of materials like polymers, plastics, and pharmaceuticals are heavily influenced by the polarity of their constituent molecules. This knowledge enables scientists to design materials with specific properties tailored for diverse applications.
The Future of Molecular Polarity
The field of molecular polarity is constantly evolving, with new discoveries and advancements continually shaping our understanding of these fundamental concepts.
Advanced Techniques
Researchers are developing advanced techniques for analyzing molecular polarity, including high-resolution spectroscopy, theoretical modeling, and computational simulations. These tools provide more accurate and detailed insights into the structure and behavior of molecules, paving the way for groundbreaking discoveries in diverse fields.
Emerging Applications
The applications of molecular polarity are expanding rapidly, with researchers harnessing this knowledge to develop innovative solutions for global challenges. For instance, understanding molecular polarity is crucial for designing new catalysts for sustainable energy production, developing targeted drug delivery systems, and creating advanced materials for clean water filtration.
Molecular Structure Lab Report Determining Polarity
Conclusion
Understanding molecular polarity is essential for anyone interested in chemistry, biology, materials science, or any field where molecular interactions are crucial. Through laboratory experiments and careful analysis, we can unveil the secrets hidden within the structure of molecules, paving the way for scientific breakthroughs and technological advancements that benefit society. So, embrace the challenge, embark on your own molecular structure investigations, and contribute to the growing body of knowledge surrounding this fascinating aspect of our world.





