I remember my first foray into the fascinating world of titration. Back in high school chemistry, I was tasked with determining the concentration of an unknown acid solution. We had been warned about the intricacies of the process, but nothing could prepare me for the thrill of watching the indicator change color, signaling the endpoint of the reaction. It was like witnessing a chemical magic trick, revealing a hidden truth about the unknown solution. This experience sparked my passion for chemistry, and I realized that titration is a powerful tool used in various fields like pharmaceuticals, environmental monitoring, and even brewing beer!
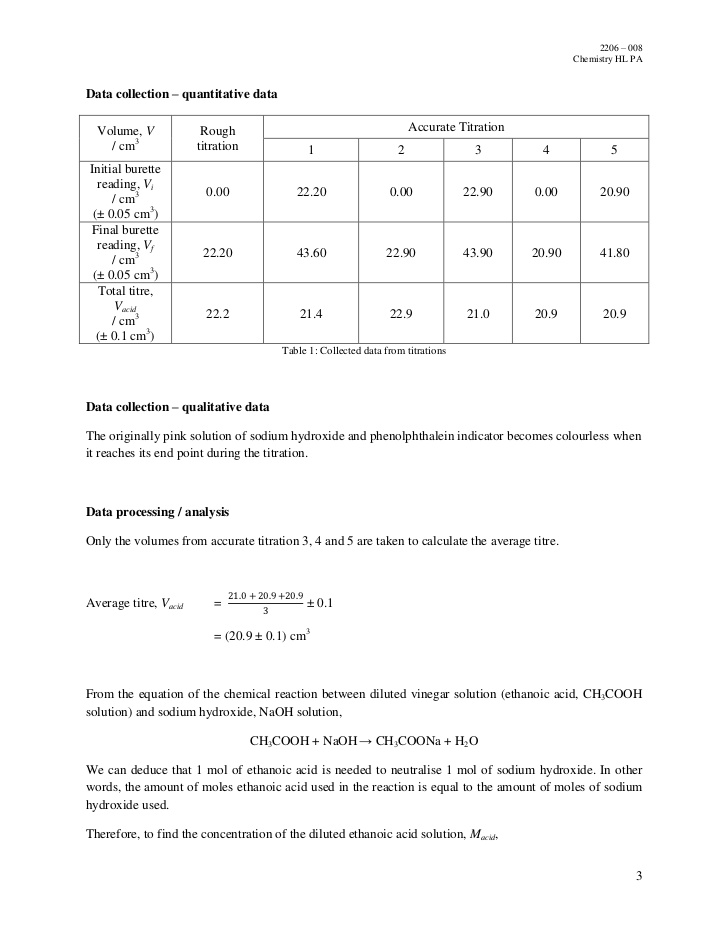
Image: www.poshpooch.ca
Titration is a fundamental technique in chemistry that allows us to determine the unknown concentration of a solution by reacting it with a solution of known concentration. This technique is incredibly versatile and has numerous applications in various scientific and industrial fields. In this lab report, we’ll delve into the fundamentals of titration, explore the different types of titrations, and discuss the common applications of this invaluable analytical technique.
Understanding the Fundamentals of Titration
Titration involves the gradual addition of a solution of known concentration, called the titrant, to a solution of unknown concentration, called the analyte. The reaction between the titrant and the analyte is typically a neutralization reaction, where an acid reacts with a base, or a redox reaction, where electrons are transferred between the reactants. We carefully monitor the reaction by observing the color change of an indicator or by measuring the pH of the solution.
The endpoint of the titration marks the point where the reaction is complete, and the titrant has completely neutralized the analyte. This is usually indicated by a distinct color change or a rapid shift in pH. At the endpoint, we can calculate the exact volume of titrant added, allowing us to determine the unknown concentration of the analyte using stoichiometry.
Types of Titration
Titration methods are categorized based on the type of reaction occurring and the nature of the titrant and analyte. Here are some of the most common types of titrations:
1. Acid-Base Titration
This is among the most widely used titrations and involves the reaction of an acid with a base. The aim is usually to determine the concentration of a strong acid or base, or to identify the unknown concentration of a weak acid or base. Acid-base titrations commonly use indicators like phenolphthalein, which changes color at a specific pH range, to signal the endpoint.
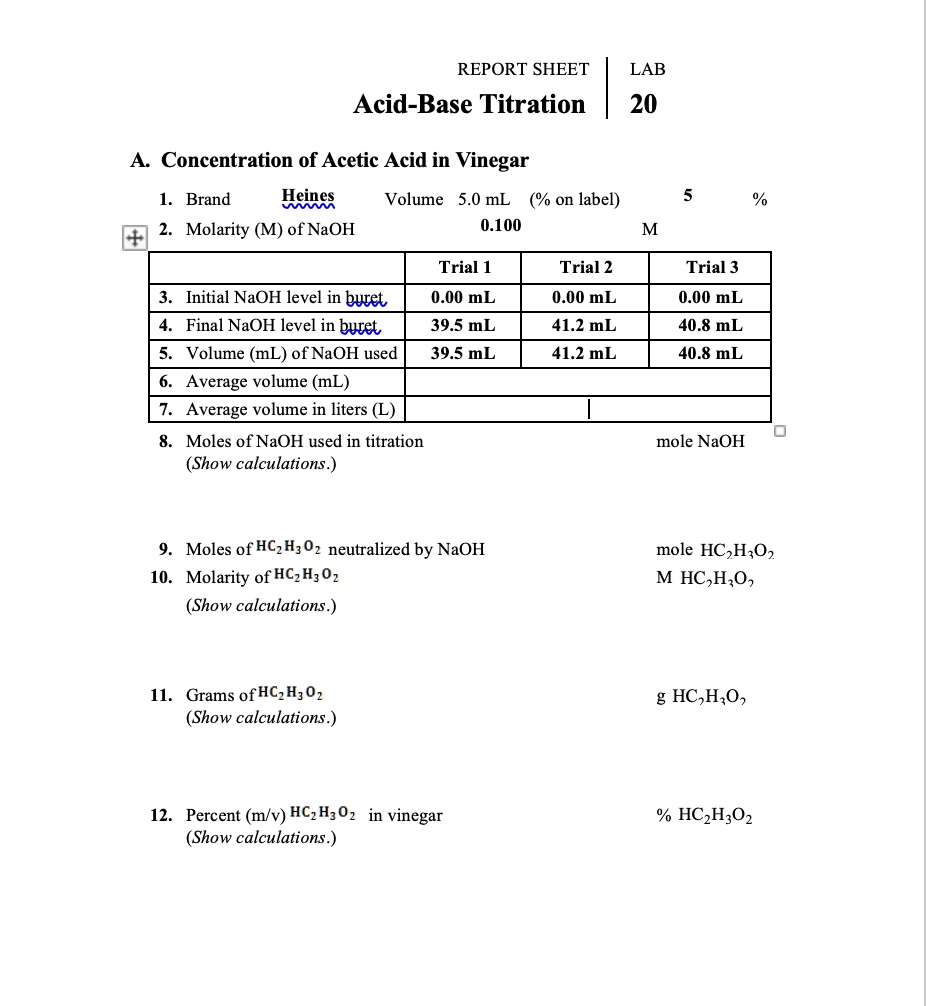
Image: www.numerade.com
2. Redox Titration
This type of titration involves a reaction between an oxidizing agent and a reducing agent. Redox titrations are often used to determine the concentration of oxidizing or reducing agents in a solution. Potassium permanganate (KMnO4) and iodine (I2) are frequently used as titrants in redox titrations.
3. Complexometric Titration
This type of titration involves the formation of a complex between a metal ion and a titrant, which is a chelating agent. These titrations are used to determine the concentration of metal ions in a solution. A common example is the use of EDTA (ethylenediaminetetraacetic acid) as a titrant to determine the concentration of calcium ions in water samples.
4. Precipitation Titration
This titration involves the formation of a precipitate between the titrant and the analyte. The endpoint is usually determined by the appearance of a turbidity (cloudiness) in the solution. Silver nitrate (AgNO3) is a common titrant used in precipitation titrations to determine the concentration of chloride ions (Cl–).
Performing a Titration: Step-by-Step Guide
Now that we’ve explored the different types of titrations, let’s walk through the steps of performing a standard titration:
- Gather your equipment: You’ll need a burette, a volumetric flask, a beaker, a pipet or a volumetric pipette, a graduated cylinder, an Erlenmeyer flask, a magnetic stirrer, a stirring bar, a pH meter or an indicator, and the titrant and analyte solutions.
- Prepare the titrant: Carefully prepare your titrant solution with the exact known concentration.
- Prepare the analyte: Calculate the volume of the analyte solution you need and transfer it into the Erlenmeyer flask.
- Fill the burette: Fill the burette with the titrant solution. Before you start titrating, it’s essential to make sure that the tip of the burette is filled with titrant and any air bubbles are removed.
- Add the indicator (optional): For acid-base titrations, you will add a few drops of an indicator to the analyte solution in the Erlenmeyer flask. Choose an indicator that changes color at the desired pH range.
- Titrate slowly: Add the titrant slowly to the analyte solution, while constantly stirring. Watch the solution carefully for any color change, indicating the endpoint.
- Read the burette: Record the exact volume of titrant used to reach the endpoint.
- Repeat the titration: Repeat the titration process at least two more times to ensure accurate and reliable results.
Example: Titration of a Weak Acid with a Strong Base
Let’s say we want to determine the concentration of a solution of acetic acid (CH3COOH), a weak acid, using a strong base such as sodium hydroxide (NaOH). Here’s a step-by-step guide to performing this titration:
- Prepare a standard solution of NaOH: This typically involves dissolving a specific mass of NaOH in a known volume of water, ensuring precise weighing and proper mixing.
- Prepare the acetic acid solution: Pipet a specific volume of the unknown acetic acid solution into the Erlenmeyer flask.
- Add indicator: Add a few drops of phenolphthalein to the acetic acid solution. Phenolphthalein is colorless in acidic solution but turns pink in basic solution.
- Titrate: Slowly add the NaOH solution from the burette to the acetic acid solution while stirring, observing for a faint pink color that persists for at least 30 seconds.
- Record the volume: Carefully note the volume of NaOH used to reach the endpoint.
- Calculate the concentration: Use the volume of NaOH and the known concentration of NaOH to calculate the concentration of acetic acid based on stoichiometry.
Latest Trends and Developments in Titration
The field of titration is constantly evolving. Researchers are developing innovative titrant systems and new techniques to enhance the accuracy, efficiency, and automation of titration processes. Here are some of the latest trends:
Automated Titration Systems
Automated titration systems using robotics and computer control have gained immense popularity in laboratories. These systems can perform titrations with greater precision and speed, significantly reducing manual labor and potential human error. They also allow for the collection and analysis of data more efficiently.
Miniaturized Titration
Researchers are working on developing miniaturized titration systems using microfluidic chips. These chips offer numerous advantages, such as reduced reagent consumption, faster analysis times, and higher throughput. They are also particularly useful for analysis of small volumes, such as in environmental monitoring and clinical chemistry.
Electrochemical Titration
Electrochemical titration employs sensors to measure changes in electrical potential or current as the titration progresses. This technique offers a more sensitive method for determining the endpoint of the titration, particularly useful for analyzing dilute solutions.
Tips and Expert Advice for Performing Titration
Performing titration requires attention to detail and careful handling of reagents. Here are some tips for successful titration:
- Use clean glassware: Any residue on glassware can affect the accuracy of your results. Always thoroughly wash all glassware with distilled water and rinse it with the solution you will be using in your titration.
- Avoid air bubbles in the burette: Ensure that the tip of the burette is filled with titrant and no air bubbles are trapped in the burette. Air bubbles can lead to inaccurate volume readings.
- Titrate slowly and carefully: Add the titrant dropwise, especially near the endpoint. Titrating too quickly can lead to overshooting the endpoint and making it difficult to achieve accurate results.
- Use proper indicator: Choose an indicator that changes color at the desired pH range for the reaction you are performing.
- Maintain a consistent stirring rate: Ensure constant stirring to ensure the titrant and analyte are well mixed throughout the titration.
- Repeat the titration: Repeat the titration at least two more times to ensure accurate and reliable results.
- Proper disposal: Titrating solutions can be hazardous. Dispose of them responsibly and follow any local environmental regulations.
FAQs
What is the purpose of an indicator in titration?
An indicator is a substance that changes color near the endpoint of a titration. This color change helps us visually identify when the reaction has reached completion. For acid-base titrations, the indicator changes color at a specific pH range, typically within the equivalence point.
What is the difference between the endpoint and the equivalence point in titration?
The **endpoint** of a titration marks the point where the indicator signals the reaction is complete, usually indicated by a color change. This is the point we visually observe. The **equivalence point** is the point where the titrant and analyte are chemically equivalent, in other words, they have reacted in the exact stoichiometric ratio. In an ideal scenario, the endpoint and equivalence point should coincide, but often there is a slight difference due to the indicator’s pH range and the inherent inaccuracy of visual observation.
What are some common errors that can occur during titration?
Common errors in titration can include:
- Improper calibration of glassware: Using uncalibrated glassware can lead to inaccurate volume measurements.
- Air bubbles in the burette: Air bubbles can lead to inaccurate volume readings and impact the final calculated concentration.
- Overshooting the endpoint: Adding the titrant too quickly can lead to overshooting the endpoint, making it difficult to obtain accurate results.
- Incorrect choice of indicator: Using an indicator that changes color outside the desired pH range can lead to inaccurate endpoint determination.
What are some applications of titration?
Titration is a versatile technique with numerous applications in various fields, including:
- Pharmaceutical industry: Titration is used to determine the concentration of active ingredients in medications, ensuring quality control and consistency.
- Food industry: Titration is essential for determining the acidity of foods and beverages, helping to maintain quality and safety.
- Environmental monitoring: Titration is used to monitor water quality by determining the concentrations of pollutants, such as acids, bases, and heavy metals.
- Chemical analysis: Titration is a cornerstone of analytical chemistry, used to determine the concentration of a wide array of chemicals in various samples.
Lab Report On Titration Of Acids And Bases
Conclusion
Titration is a powerful analytical technique that allows us to determine the unknown concentration of a solution. Mastering titration requires careful planning, meticulous execution, and a thorough understanding of the underlying principles. By carefully following the steps outlined in this lab report, you can confidently perform titrations and use this invaluable technique in your own scientific endeavors. Remember to practice, experiment, and stay curious. The world of chemistry holds countless fascinating mysteries just waiting to be uncovered!
Are you interested in this topic? Let us know in the comments below!





