Have you ever wondered how your cells stay hydrated, or how plants draw water from the soil? The answer lies in a fascinating process called osmosis, and the Amoeba Sisters are here to break it down for us with their engaging and informative videos.
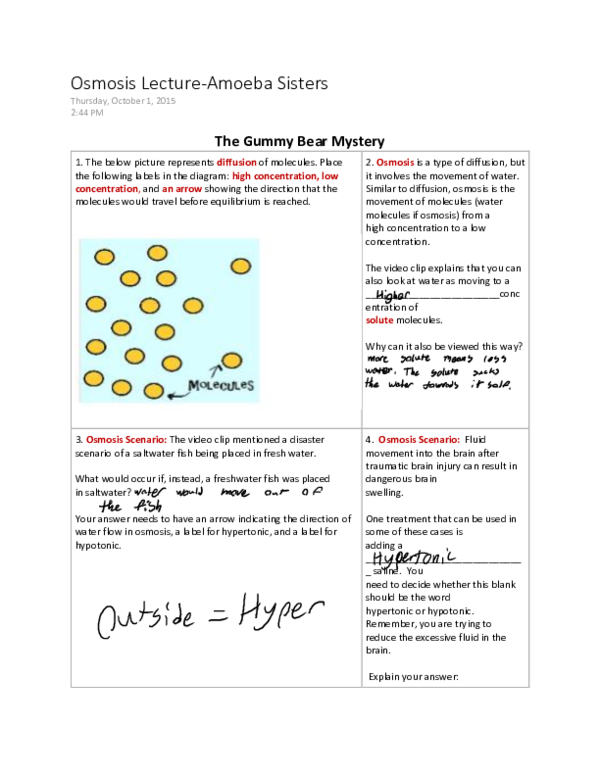
Image: worksheetfullebersr.z13.web.core.windows.net
Osmosis is the movement of water across a selectively permeable membrane, from an area of high water concentration to an area of low water concentration. This process is crucial for maintaining the balance of fluids and solutes within cells and organisms. In this article, we’ll delve deeper into the world of osmosis, exploring its key concepts, real-world examples, and answering common questions addressed by the Amoeba Sisters.
Osmosis: A Journey Through Membranes
Understanding Selective Permeability
To grasp osmosis, we must first understand the concept of a selectively permeable membrane. Imagine a wall with doors that open and close based on what’s trying to pass through. A selectively permeable membrane, like the cell membrane, acts similarly, allowing certain molecules to pass through while blocking others. In the case of osmosis, water molecules are the primary players, effortlessly slipping in and out of the membrane, while larger molecules like sugars and proteins are kept at bay.
The Driving Force: Concentration Gradients
Think of a crowded room where people want to move to a less crowded space. This natural desire to spread out applies to water molecules as well. Water molecules move from an area where they are more concentrated (like fresh water) to areas where they are less concentrated (like salt water). This difference in concentration is known as a concentration gradient, the driving force behind osmosis.

Image: diffuserdian.blogspot.com
Osmosis in Action: Real-World Examples
Let’s look at some real-world examples to understand how osmosis plays out in our world:
- Saltwater Fish: Saltwater fish live in a hypertonic environment, meaning the concentration of salts outside their bodies is higher than inside. To compensate, they constantly drink water and excrete concentrated urine, relying on osmosis to keep their internal balance.
- Plant Cells: Plants get their water through their roots, thanks to osmosis. Water molecules move from the soil, which is hypotonic to the cells, into the plant’s roots, allowing them to grow and thrive.
- Red Blood Cells: Red blood cells need to maintain a specific water content to function properly. If they are placed in a hypotonic solution (with a lower concentration of solutes), they will absorb water and potentially burst. In a hypertonic solution, they will lose water and shrink. This is why it’s crucial to stay hydrated, ensuring our red blood cells have just the right amount of water.
Key Terms Explained
Hypotonic, Hypertonic, and Isotonic
These terms help us classify solutions based on their relative solute concentration, which is crucial for understanding osmosis:
- Hypotonic Solution: A solution with a lower concentration of solutes than another solution. Water will tend to move into the hypotonic solution.
- Hypertonic Solution: A solution with a higher concentration of solutes than another solution. Water will tend to move out of the hypertonic solution.
- Isotonic Solution: Two solutions with equal concentrations of solutes. There is no net movement of water between them.
Osmotic Pressure
Osmotic pressure is the force that needs to be applied to stop the flow of water across a semi-permeable membrane. In simpler terms, it’s the pressure exerted by the water molecules as they try to move from one area to another. Higher osmotic pressure indicates a stronger force driving water movement.
Amoeba Sisters: Bringing Osmosis to Life
The Amoeba Sisters are known for their engaging and accessible videos that simplify complex biological concepts, making them enjoyable for learners of all ages. Their video recap on osmosis offers a clear and concise explanation, coupled with visually appealing animations and relatable examples.
The Amoeba Sisters cover essential points in their video, such as:
- Visualizing Osmosis: The video uses animated examples of water molecules moving across a membrane, helping viewers visualize the process vividly.
- Simple Analogy: The sisters employ an analogy involving a sugar-filled balloon to illustrate the concept of concentration gradients and the direction of water movement.
- Real-World Examples: They connect osmosis to everyday phenomena like wilted plants and why we need to drink water.
- Important Notes: The sisters highlight key points, such as the impact of solute concentration, the direction of water movement, and its importance in biological systems.
Amoeba Sisters Video Recap Osmosis Answers
Beyond the Basics: Exploring Osmosis Further
Osmosis isn’t just about water moving around cells; it plays a crucial role in many biological phenomena:
- Plant Growth: Osmosis is essential for the transportation of water from roots to leaves, which is vital for photosynthesis and plant growth.
- Kidney Function: The kidneys use osmosis to filter blood, removing waste products while retaining essential nutrients and water.
- Cell Signaling: Osmosis influences ion movement across cell membranes, impacting cellular communication and responses to external stimuli.
Understanding osmosis is fundamental to grasping many biological processes. The Amoeba Sisters’ video recap provides a solid foundation, inspiring further exploration of this fascinating phenomenon. Now, armed with the knowledge from this recap and the Amoeba Sisters’ guidance, you can take a deeper dive into the world of osmosis and its impact on life.





