Have you ever wondered why water is a liquid at room temperature while methane is a gas? The answer lies in the unseen world of intermolecular forces, the gentle dance of attraction and repulsion between molecules that shapes the world around us. These forces, influenced by the polarity of molecules, dictate everything from the boiling point of a substance to the structure of proteins. But understanding these concepts can feel like navigating a labyrinth of confusing definitions and complex diagrams. Fear not! This guide will unravel the mysteries of polarity and intermolecular forces, making these concepts crystal clear through a hands-on exploration with the help of online Gizmos.
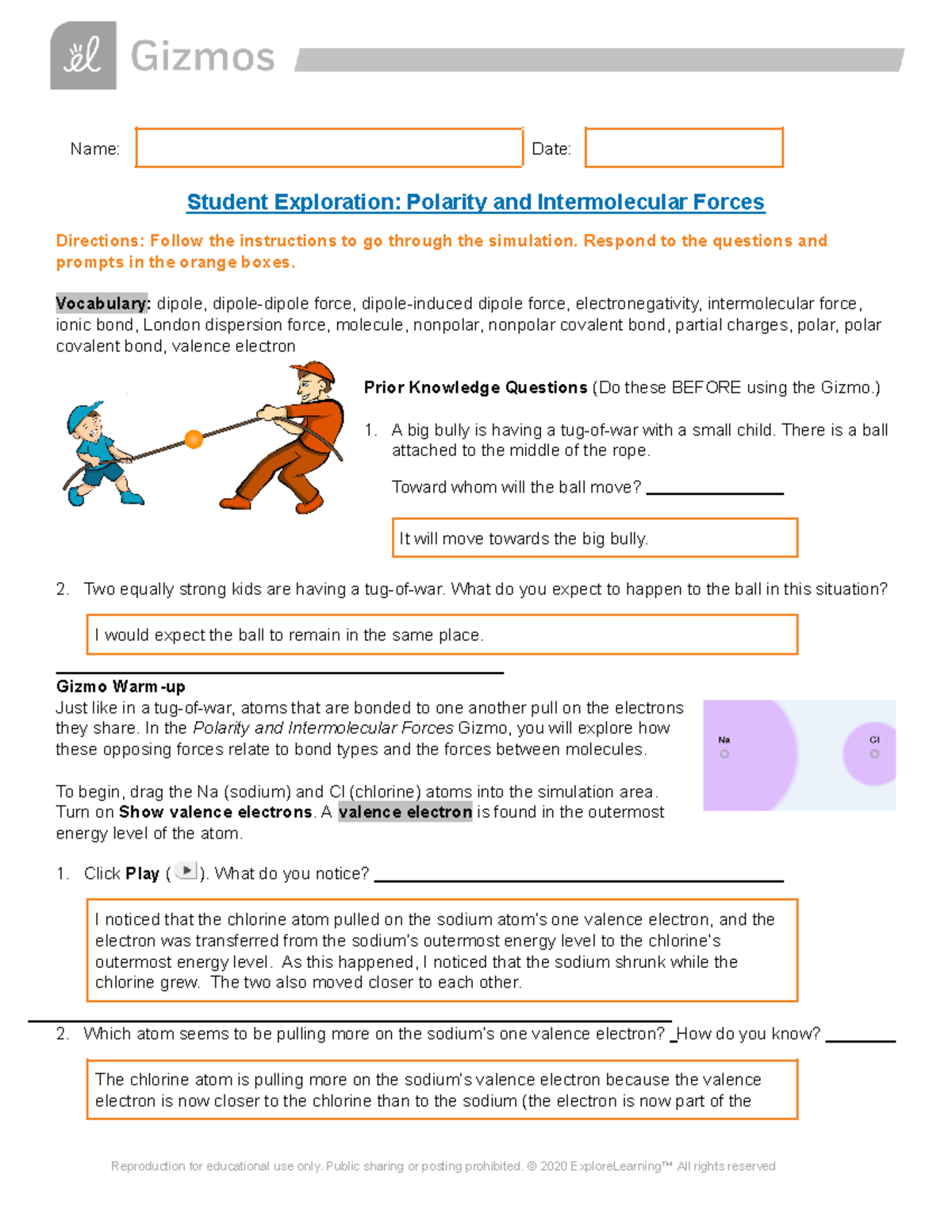
Image: www.studocu.com
Imagine yourself as a tiny molecule, zipping around in a bustling world of other molecules. You’re constantly bumping into each other, sometimes sticking together, sometimes bouncing off. This intricate ballet of molecular interactions is governed by the principles of polarity and intermolecular forces. While the individual molecules themselves might be too small to see, their behavior and the properties they exhibit, from the water we drink to the air we breathe, are directly influenced by these invisible forces. These Gizmos will serve as virtual microscopes, allowing us to zoom into this microscopic world and witness the delicate dance that shapes our macroscopic reality.
Delving into the Core Concepts: Polarity and Intermolecular Forces
At the heart of this molecular dance lies the concept of polarity. Imagine a molecule as a tiny tug-of-war between opposing forces – positive and negative charges. If these forces are distributed evenly, the molecule is considered nonpolar, like a perfectly balanced rope. But, if the forces are unevenly distributed, creating a positive and a negative end, the molecule becomes polar – like a tug-of-war where one team is stronger than the other. Think about water, a molecule composed of two hydrogen atoms and one oxygen atom. Oxygen is more electronegative than hydrogen, meaning it pulls the electrons closer to itself, creating a slight negative charge around oxygen and a slight positive charge around hydrogen. This creates a dipole, a separation of charge within the molecule, making water a polar molecule.
Now that we understand the concept of polarity, it’s time to explore the different types of intermolecular forces, the attractive interactions between molecules. These forces, while weaker than the intramolecular forces (the bonds within a molecule), are the driving force behind many fascinating phenomena. The strength of these forces determines a substance’s physical properties like boiling point, melting point, and viscosity.
Dipole-Dipole Forces: The Dance of Opposite Charges
Think of these forces as a magnetic pull between two polar molecules. The positively charged end of one molecule attracts the negatively charged end of another molecule, resulting in a gentle attraction between them. The stronger the dipole moment (the separation of charges within a molecule), the stronger the dipole-dipole forces. This is why, for instance, acetone, which possesses a larger dipole moment than water due to the presence of a more electronegative oxygen atom, has a higher boiling point than water.
Hydrogen Bonding: The Strongest Bond
Among the intermolecular forces, hydrogen bonding stands out as the strongest. A special type of dipole-dipole force, it occurs specifically between a hydrogen atom covalently bonded to a highly electronegative atom (like oxygen, nitrogen, or fluorine) and an electronegative atom in another molecule. This strong bond arises from the highly polarized nature of the hydrogen atom, creating a strong attraction to the electronegative atom in another molecule.

Image: www.studypool.com
London Dispersion Forces: The Temporary Attraction
Even nonpolar molecules experience a fleeting attraction – London Dispersion forces. These forces arise from temporary fluctuations in the electron distribution within a molecule. Although the electrons are evenly distributed on average, at any given time, they can briefly shift, creating a temporary dipole moment. This momentary shift in charge can induce a dipole in a nearby molecule, leading to a weak attraction. The strength of London Dispersion forces depends on the size and shape of the molecule; larger molecules have greater electron clouds, leading to stronger interactions.
Visualizing the Interplay: Gizmos in Action
To grasp the nuances of polarity and intermolecular forces, it’s crucial to visualize their effects. This is where online Gizmos, interactive simulations that bring science to life, come in handy. With these Gizmos, you can manipulate variables, observe the changes in molecular behavior, and gain a deeper understanding of the invisible forces at play.
Exploring Dipole Moments: The Virtual Tug-of-War
One helpful Gizmo allows you to explore dipole moments by creating different molecules. You can alter the types of atoms present and their arrangement to see how the electron distribution and dipole moment change. Seeing how the dipole moment alters based on the arrangement and polarity of the individual bonds within the molecule is an insightful exercise.
Investigating Intermolecular Forces: A Molecular Dance Floor
Another powerful Gizmo showcases the effect of different intermolecular forces on the properties of liquids. You can switch between various substances like water, acetone, and methane and observe how they behave. You’ll learn to see how the attractive forces between molecules impact their boiling point, melting point, and surface tension. Watching water molecules form strong hydrogen bonds, making it difficult to break at room temperature, contrasting with the weak London Dispersion forces in methane, resulting in its gaseous state, is a visual journey into the world of intermolecular forces.
Mastering the Molecular Dance: Tips for Success
Understanding the concepts of polarity and intermolecular forces is an essential building block for deeper exploration in chemistry. Here are some tips to help you master this captivating world:
- Build a Framework: Start by defining the key terms involved, like electronegativity, dipole moment, and the different types of intermolecular forces.
- Visualize: Don’t just memorize definitions – imagine the molecules interacting. Use Gizmos, online drawings, or even create physical models to represent the behavior of molecules and the forces acting upon them.
- Connect to Real-world Phenomena: Think about how the concepts you’re learning apply to your everyday experiences. Why does water dissolve sugar but not oil? Why does ice float? These everyday wonders are a manifestation of the concepts you’re studying!
Polarity And Intermolecular Forces Gizmos Answer Key
Conclusion: A World of Endless Wonder
The world of polarity and intermolecular forces might seem abstract at first, but it holds the key to understanding the behavior of everything around us. With online Gizmos, we can explore this invisible world, witnessing the intricate dance of molecules and the forces that govern their interactions. By embracing these concepts, we gain a deeper appreciation for the fundamental principles that shape the world we experience. So, delve into the mysteries of polarity and intermolecular forces, explore the wonders of Gizmos, and discover a world of endless wonder hidden within the seemingly simple molecules around us.





