Have you ever wondered why lemons taste sour, or why soap feels slippery? These everyday experiences are all connected to a fundamental concept in chemistry: acids, bases, and solutions. This fascinating realm governs a wide range of chemical reactions and processes, shaping the world around us.
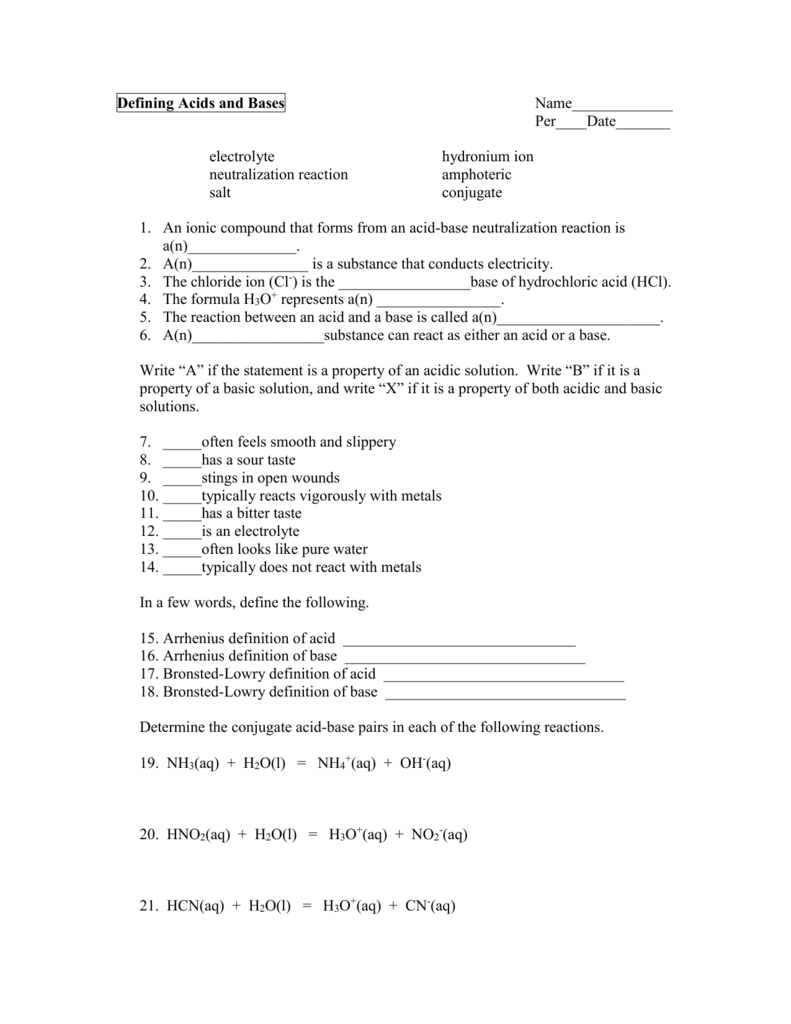
Image: worksheetlistlemann.z13.web.core.windows.net
Today, we will embark on a journey to explore the world of acids, bases, and solutions. We’ll delve into the core principles, uncover their surprising applications, and provide you with all the answers to your burning questions. Whether you’re a student preparing for an exam, a curious individual seeking knowledge, or simply eager to understand the chemistry that surrounds you, this comprehensive guide has everything you need.
Delving into the Fundamentals: Acids, Bases, and Solutions
Before we dive into the nitty-gritty of the review, let’s establish a solid foundation of understanding. Let’s start with defining our key players:
Acids: You probably know them as sour substances like lemon juice or vinegar. But what makes them so? Acids are compounds that release hydrogen ions (H+) when dissolved in water. Think of them like tiny proton donors, giving up their positive charge to the solution. Their acidic nature is often measured using the pH scale, with low pH values indicating higher acidity.
Bases: These compounds are the opposites of acids. They release hydroxide ions (OH-) when dissolved in water. Imagine bases as proton acceptors, sopping up those pesky hydrogen ions. They are often characterized by being slippery and bitter-tasting. Higher pH values on the scale reflect greater basicity.
Solutions: A solution is a homogeneous mixture of two or more substances. One key ingredient is the solvent – usually a liquid that dissolves the other components called solutes. The classic example is salt dissolving in water, forming a saltwater solution.
Mastering the Concepts: A Review of Key Ideas
Now, let’s get down to business and dive into those important concepts that are often tested in review sessions.
1. The pH Scale: This scale is a visual representation of acidity and basicity. Values from 0 to 7 indicate acidic solutions, with 0 being the most acidic. Neutral solutions like pure water have a pH of 7. Values from 7 to 14 indicate basic solutions, with 14 being the most basic. Remember, the pH scale is logarithmic, so each unit represents a tenfold change in acidity or basicity.
2. Neutralization Reactions: This is where acids and bases meet, and magic happens! When an acid and base react, they neutralize each other, forming salt and water. The salt is a compound made of the positive ion from the base and the negative ion from the acid. This is why we often use antacids (bases) to neutralize the “acidic” discomfort of heartburn.
3. Indicators: These special substances change color depending on the pH of a solution. They help us visually determine whether a solution is acidic, basic, or neutral. Think about litmus paper, which turns red in acids and blue in bases. Other common indicators include phenolphthalein, which turns pink in basic solutions, and methyl orange, which turns red in acidic solutions.
4. Titration: This technique is used to determine the concentration of an unknown acid or base. It involves slowly adding a solution of known concentration (a titrant) to an unknown solution until neutralization is reached. A pH meter or indicator is used to monitor the pH change during the titration process.
5. Strong and Weak Acids and Bases: Acids and bases can be classified as strong or weak depending on their ability to ionize in water. Strong acids and bases completely ionize in water, meaning they dissociate into their constituent ions to a much greater extent. Weak acids and bases only partially ionize, meaning they release a smaller number of ions into solution.
6. Common Examples: To solidify your understanding, let’s explore some real-world examples of acids and bases.
Acids:
- Hydrochloric acid (HCl): Found in stomach acid, essential for digestion.
- Sulfuric acid (H2SO4): Used in car batteries, fertilizer production.
- Acetic acid (CH3COOH): Gives vinegar its tart flavor.
Bases:
- Sodium hydroxide (NaOH): Used in soap and drain cleaner.
- Calcium hydroxide (Ca(OH)2): Used in cement and mortar.
- Ammonia (NH3): Found in household cleaners.
Real-world applications: The Chemistry that Shapes Our World
Acids, bases, and solutions are not just theoretical concepts; they play a vital role in our daily lives, impacting various industries and processes.
1. Food and Beverage: You’ve already encountered acids in your kitchen. Think of citrus fruits, vinegar, and wine. Bases are also crucial, as baking soda (sodium bicarbonate) is a common leavening agent in baking.
2. Medicine: Many drugs and medications are either acids or bases. Antacids, for example, are bases used to relieve heartburn. Aspirin (acetylsalicylic acid) is a familiar example of an acidic drug.
3. Agriculture: Acids and bases are essential for soil fertility and crop growth. Lime (calcium oxide) is a basic substance used to neutralize acidic soil and improve its pH.
4. Environmental Protection: Acids and bases play a role in controlling water quality and reducing pollution. Acid rain, for instance, is a consequence of industrial emissions, and it can damage ecosystems.
5. Industrial Processes: Acids and bases are fundamental to numerous manufacturing processes, including the production of plastics, fertilizers, and pharmaceuticals.

Image: tempojs.com
Expert Insights: Tips for Mastery
Now that you’ve gained a solid understanding of acids, bases, and solutions, let’s tap into expert advice to enhance your learning journey.
- Visualize the Concepts: Draw diagrams or use models to visualize the interactions between molecules and ions in solution.
- Practice, Practice, Practice: Work through practice problems and review questions to solidify your grasp of the concepts.
- Seek Help When Needed: Don’t hesitate to ask questions or seek help from your teacher, tutor, or online resources.
- Connect to the Real World: Look for applications of acids, bases, and solutions in your everyday life to make learning more engaging.
Acids Bases And Solutions Review Answer Key
Conclusion: Embark on Your Chemical Adventure!
Congratulations! You’ve delved into the fascinating world of acids, bases, and solutions, mastering key concepts, exploring real-world applications, and receiving valuable insights from experts. Remember, learning chemistry doesn’t have to be a daunting process. Embrace the journey, stay curious, and celebrate the power of understanding the chemistry that shapes our world.
Now, it’s your turn to take action! Share your experiences and insights in the comments below. Let’s continue the conversation and make chemistry even more accessible and engaging for everyone.





