Remember those confusing chemistry diagrams from high school? The ones with dots and lines scattered around a central atom? Those are Lewis dot diagrams, and they hold the key to understanding how elements bond with each other. While they may seem daunting at first glance, mastering Lewis dot diagrams empowers you to visualize and predict chemical interactions.
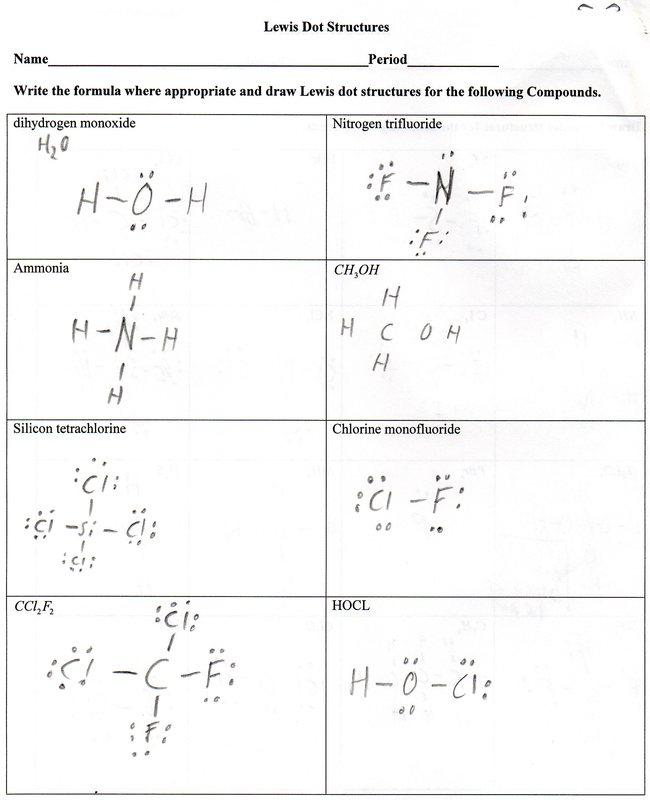
Image: mydiagram.online
From understanding simple molecule formation to predicting the behavior of complex compounds, Lewis dot diagrams are a valuable tool for anyone seeking a deeper understanding of chemical bonding. In this article, we’ll delve into the world of Lewis dot diagrams, explore their importance, and equip you with the knowledge to confidently tackle any Lewis dot diagram worksheet.
What are Lewis Dot Diagrams?
Lewis dot diagrams, also known as electron dot diagrams, are simplified representations of the valence electrons of an atom, which are the electrons in the outermost shell. These diagrams use dots to symbolize the valence electrons and a symbol for the element in question. The placement of the dots is crucial, as it visually depicts the distribution of electrons around the atom, which plays a critical role in chemical bonding.
Let’s break it down: Each dot surrounding the element’s symbol represents a single valence electron. The diagram follows a specific arrangement, accommodating up to eight dots around the symbol. This arrangement is based on the principle that atoms tend to gain, lose, or share electrons to achieve a stable octet configuration, meaning they have eight valence electrons, like noble gases.
Deciphering the Dots: How to Draw Lewis Dot Diagrams
Drawing a Lewis dot diagram might seem like a daunting task, but it’s actually quite straightforward. Follow these simple steps, and you’ll be drawing Lewis dot diagrams like a pro in no time.
1. Determine the Element’s Valence Electrons
The first step is to identify the number of valence electrons for the element you are working with. This can be easily determined by referring to the element’s position on the periodic table. The group number of an element, ignoring the transition metals, corresponds to its valence electron count.

Image: wordworksheet.com
2. Place the dots around the element’s symbol
Once you know the number of valence electrons, you start placing dots around the element’s symbol, following a specific pattern. Start by placing one dot on each side of the symbol (north, east, south, west). If you have more than four valence electrons, add a second dot on each side, filling each side with two dots.
3. Consider the octet rule
The octet rule is a key principle in Lewis dot diagrams. It states that elements tend to gain, lose, or share electrons to achieve a stable octet configuration, meaning they have eight valence electrons, just like noble gases. This rule helps predict how elements will bond with each other.
Understanding Chemical Bonding through Lewis Dot Diagrams
Lewis dot diagrams are not just pretty drawings; they are powerful tools for understanding how atoms bond to form molecules. By depicting valence electrons, Lewis dot diagrams provide a visual representation of the sharing or transferring of electrons during bonding.
Ionic Bonding
In ionic bonding, one atom loses electrons, becoming a positively charged ion (cation), while another atom gains those electrons, becoming a negatively charged ion (anion). The Lewis dot diagrams for ionic bonding show the transfer of electrons from the cation to the anion, resulting in the formation of an ionic compound. For example, when sodium (Na) reacts with chlorine (Cl), sodium loses its valence electron, forming Na+, while chlorine gains that electron, forming Cl-. This transfer of electrons is represented in their Lewis dot diagrams, emphasizing the formation of the ionic bond between sodium and chlorine.
Covalent Bonding
Covalent bonding involves the sharing of electrons between atoms. Lewis dot diagrams are essential for visualizing covalent bonding. The shared electrons are represented as lines connecting the bonded atoms, while lone pairs—electrons not involved in bonding—remain as dots on the atoms. For example, in the molecule of water, H2O, oxygen shares two electrons with each hydrogen atom, resulting in two single covalent bonds. This sharing is clearly depicted in the Lewis dot diagram for water, indicating the formation of two covalent bonds and two lone pairs on the oxygen atom.
Lewis Dot Diagram Worksheet: Practice Makes Perfect
The best way to master the art of Lewis dot diagrams is through practice. Lewis dot diagram worksheets provide a structured framework for honing your understanding of these diagrams. Many resources are available online and in textbooks. Using these resources, you can test your skills, and clarify any confusion.
Tips and Expert Advice
Drawing Lewis dot diagrams effectively involves a few key tips. Here are some expert recommendations to make your Lewis dot diagram journey smoother.
1. Start with the Central Atom
When you are drawing Lewis dot diagrams for molecules, start with the central atom. The central atom is usually the least electronegative atom, meaning it has a weaker attraction to electrons.
2. Consider the Octet Rule
The octet rule guides the bonding of atoms. Ensure that each atom bonded, including the central atom, has a stable octet of electrons. However, there are exceptions to this rule, particularly with elements like hydrogen, helium, and beryllium, which can have duet configuration with only two electrons.
3. Don’t Forget Lone Pairs
Remember that Lewis dot diagrams also depict the lone pairs of electrons. Lone pairs are the non-bonding electrons that are not involved in bonding with other atoms. They are crucial for understanding the geometry and polarity of molecules.
FAQs About Lewis Dot Diagrams
Here are some frequently asked questions about Lewis dot diagrams:
Q: What is the significance of formal charge in Lewis dot diagrams?
A: Formal charge refers to the theoretical charge assigned to an atom in a molecule, assuming that bonding electrons are equally shared. It is a method for determining the most stable Lewis structure for a molecule. Formal charge helps us identify the most likely structure for a molecule based on the distribution of electrons.
Q: How do I know which resonance structures are more significant?
A: Resonance structures are alternative Lewis structures for a molecule that differ in the placement of electrons. The most significant resonance structures are those where the formal charge on atoms is minimized, and negative charges are placed on the most electronegative atoms.
Q: How do Lewis dot diagrams relate to VSEPR theory?
A: VSEPR theory (Valence Shell Electron Pair Repulsion theory) predicts the three-dimensional shape of molecules based on the repulsion of electron pairs around the central atom. Lewis dot diagrams provide the basis for applying VSEPR theory by depicting the number of bonding and lone pairs of electrons around the central atom.
Lewis Dot Diagram Worksheet Answer Key
https://youtube.com/watch?v=XYApht-9ybM
Conclusion
Lewis dot diagrams are a fundamental tool in understanding chemical bonding and predicting molecular structures. By mastering the art of drawing and interpreting these diagrams, you can unlock a deeper understanding of the world around us. So, are you ready to take on the challenge of Lewis dot diagram worksheets? Let’s dive into the world of chemical bonding!





