Picture this: you’re staring down the barrel of a Chemistry Unit 3 exam, your mind a jumble of electrons, orbitals, and bonding theories. The pressure mounts, the anxiety builds, and you desperately search for that missing piece of knowledge that will unlock the secrets of the atom. Fear not, for this guide is your key to conquering Chemistry Unit 3 and emerging as a champion of the atomic realm.
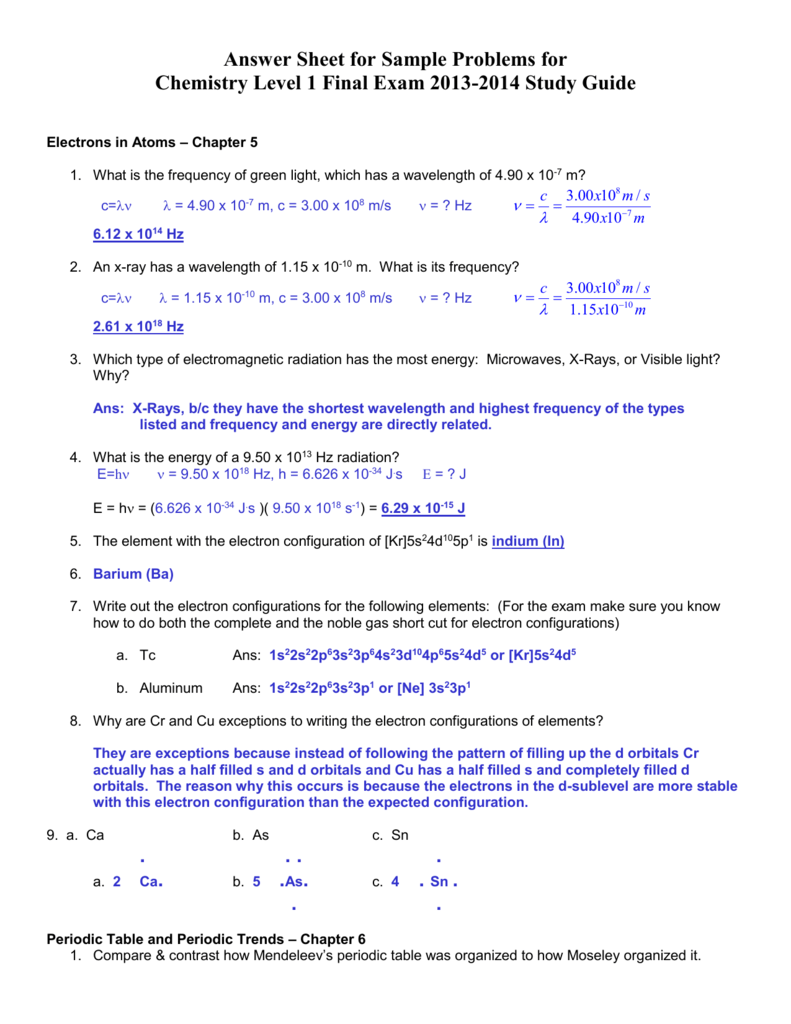
Image: studylib.net
Unit 3 in Chemistry dives deep into the heart of the atom, exploring its structure, behavior, and the fascinating ways it interacts with other atoms. This journey will lead you through the intricacies of electron configuration, delve into the mysteries of chemical bonding, and equip you with the tools to predict the properties of molecules. It’s a crucial step in your scientific journey, building a foundation for countless future discoveries and applications.
The Intricate Dance of Electrons: Unveiling Electron Configuration
Understanding electron configuration is like learning the choreography of an intricate atomic ballet. Every atom has its unique way of arranging its electrons in specific energy levels and orbitals. This arrangement dictates how the atom will interact with other atoms, shaping the foundations of chemical bonds.
At the heart of this ballet lies the quantum mechanical model, a revolutionary approach that abandons the deterministic orbits of Bohr’s model. Here, electrons exist in probability clouds, represented by atomic orbitals, which describe their spatial distribution around the nucleus.
The Four Quantum Numbers:
- Principal Quantum Number (n): This number represents the energy level of an electron and can take on positive integer values (1, 2, 3, etc.). As n increases, the energy level of the electron increases, and it is farther from the nucleus.
- Angular Momentum Quantum Number (l): This number describes the shape of an electron’s orbital and ranges from 0 to n-1. l=0 corresponds to an s orbital (spherical), l=1 to a p orbital (dumbbell shaped), l=2 to a d orbital (more complex shapes), and so on.
- Magnetic Quantum Number (ml): This number specifies the orientation of an orbital in space. It can take on integral values from -l to +l, including 0. For example, a p orbital (l = 1) has three possible orientations, ml = -1, 0, +1.
- Spin Quantum Number (ms): This number accounts for the intrinsic angular momentum of an electron, which is quantized and can be either spin up, ms = +1/2, or spin down, ms = -1/2.
The Aufbau Principle and Hund’s Rule:
- Aufbau Principle: Electrons are filled into orbitals in increasing order of energy, beginning with the lowest energy level.
- Hund’s Rule: Electrons will occupy orbitals individually before pairing up within the same orbital, maximizing the number of unpaired electrons.
Let’s illustrate with an example: Oxygen has an atomic number of 8. This means it has 8 protons and 8 electrons. Its electron configuration is 1s² 2s² 2p⁴. Notice how the electrons are filled according to the Aufbau principle and how Hund’s rule ensures that two of the 2p orbitals have one electron each before the third 2p orbital receives its second electron.
The Force that Binds: Decoding Chemical Bonding
With a grasp of electron configuration in hand, we can navigate the intriguing world of chemical bonding. Atoms are the building blocks of all matter, and chemical bonds are the glue that holds them together, creating molecules of incredible diversity.
Ionic Bonding: The Dance of Opposite Charges
Ionic bonds form through the transfer of electrons between atoms. Metals, with their loosely held valence electrons, readily lose them, becoming positively charged ions (cations). Nonmetals, with their tendency to gain electrons, become negatively charged ions (anions). These oppositely charged ions are powerfully attracted to one another, forming a strong electrostatic force known as an ionic bond.
Think of salt: Sodium (Na), a metal, readily loses its lone valence electron to become a positively charged ion (Na⁺). Chlorine (Cl), a nonmetal, happily accepts that electron to become a negatively charged ion (Cl⁻). These opposing charges create a strong attraction, forming the iconic NaCl crystal lattice.
Covalent Bonding: Sharing is Caring
In covalent bonding, atoms share electrons rather than completely transferring them. This sharing occurs when two nonmetals, with similar tendencies to gain electrons, join forces. By combining their electron clouds, they create a region of shared electron density, forming a strong covalent bond.
Think of water:
Two hydrogen atoms (H), with their single electron each, link with an oxygen atom (O), with six valence electrons. By sharing electrons, they achieve a stable, filled outer shell, forming the familiar H₂O molecule. The shared electrons create a region of high electron density, forming a strong covalent bond.
Metallic Bonding: A Sea of Electrons
Metallic bonding is a uniquely collaborative dance among metal atoms. Metal atoms readily lose their valence electrons, creating a “sea” of delocalized electrons that are free to move throughout the entire metal structure. These electrons act like a glue, holding the positively charged metal ions together in a lattice.
Think of copper: Copper atoms readily release their valence electrons, creating a sea of delocalized electrons that bind the copper ions together in a lattice structure. This delocalization of electrons is responsible for the unique properties of metals – their conductivity, malleability, and ductility.
Intermolecular Forces: The Subtle Bonds that Shape Our World
While chemical bonds hold atoms together within molecules, weaker forces called intermolecular forces act between molecules themselves. These forces are crucial in determining the physical properties of substances, such as melting point, boiling point, and solubility.
- Hydrogen Bonding: A Special Kind of Attraction
Hydrogen bonding is the strongest type of intermolecular force, occurring when hydrogen is bonded to a highly electronegative atom like oxygen, fluorine, or nitrogen. This creates a strong dipole moment, where the hydrogen atom becomes slightly positive and the more electronegative atom slightly negative. This results in a strong attractive force between molecules. Water, with its hydrogen bonds, is a perfect example of how this force can shape the properties of a substance.
- Dipole-Dipole Interactions: Attraction Between Polar Molecules
Molecules with polar bonds, where there is an uneven distribution of electron density, can attract each other through dipole-dipole interactions. These interactions are weaker than hydrogen bonds but still significant in determining the properties of polar molecules.
- London Dispersion Forces: Temporary Attractions Between All Molecules
London dispersion forces are the weakest type of intermolecular force, arising from temporary fluctuations in electron distribution around atoms. These weak attractions occur between all molecules, even nonpolar ones. While these forces are individually weak, they can be significant in large molecules or at low temperatures.

Image: pj-land.blogspot.com
Mastering the Secrets of Molecular Geometry: VSEPR Theory
The shape of a molecule is not simply a random arrangement of atoms. The positions of atoms within a molecule are determined by the repulsions between electrons in the valence shell, as dictated by the Valence Shell Electron Pair Repulsion (VSEPR) theory. This theory allows us to predict the geometry of a molecule based on the number of electron pairs around the central atom.
- Linear Geometry: Two electron pairs around the central atom create a linear shape, with an angle of 180 degrees between the two bonded atoms.
- Trigonal Planar Geometry: Three electron pairs around the central atom create a trigonal planar shape, with bond angles of 120 degrees.
- Tetrahedral Geometry: Four electron pairs around the central atom create a tetrahedral shape, with bond angles of 109.5 degrees.
Understanding VSEPR theory is crucial for predicting a molecule’s shape, which directly affects its properties, such as its reactivity and its ability to participate in intermolecular interactions.
A World of Molecules: The Power of Chemical Reactions
Chemical reactions are the heart of chemistry. They involve the rearrangement of atoms and molecules, breaking and forming new bonds. Understanding how reactions occur is key to understanding the world around us.
- Collision Theory: The Dance of Molecules
For a chemical reaction to occur, molecules must collide with sufficient energy, known as activation energy. This energy is required to break existing bonds and form new ones. The higher the activation energy, the slower the reaction rate.
- Factors Affecting Reaction Rate:
Several factors can influence the rate of a chemical reaction, including:
* **Temperature:** Increasing temperature provides more energy, leading to more frequent and successful collisions.
* **Concentration:** A higher concentration of reactants leads to a higher rate of collisions.
* **Surface Area:** A higher surface area of reactants provides more points of contact for collisions.
* **Catalyst:** A catalyst speeds up a reaction without being consumed itself by lowering the activation energy.Putting it All Together: Applications of Chemistry Unit 3
The knowledge gained from Chemistry Unit 3 has far-reaching applications, extending beyond the classroom into the real world.
- Pharmaceuticals: Understanding molecular structure and bonding is crucial in designing new drugs with specific properties that target certain diseases.
- Materials Science: The properties of materials are directly related to their atomic structure and bonding. This knowledge is used to create new materials with desired properties for industrial applications.
- Environmental Chemistry: Understanding chemical reactions is essential for addressing environmental issues, such as pollution control and the development of sustainable technologies.
- Biochemistry: The principles of chemistry are essential for understanding biological processes at the molecular level. This knowledge is vital for studying DNA, proteins, and enzymes.
Expert Insights and Actionable Tips
Master the Foundation:
- Don’t shy away from the fundamental concepts. Invest in understanding electron configuration, bonding, and VSEPR theory, as they form the bedrock of Unit 3.
- Practice, practice, practice! The more you engage with problems and examples, the more confident you’ll become.
Seek Help and Collaborate:
Don’t hesitate to reach out to your instructor, teaching assistants, or classmates when you encounter challenges. Collaboration and mutual support can be invaluable in navigating the intricacies of Chemistry Unit 3.
Visual Learning:
- Use visual aids like diagrams and models to grasp the abstract concepts. These representations can make complex ideas much easier to understand.
Chemistry Unit 3 Review Answer Key
A Journey of Discovery: The Next Steps
By mastering the principles of Chemistry Unit 3, you unlock a world of possibilities, not just in your academic pursuits, but also in your everyday life. You gain a deeper understanding of the materials that surround you, the chemical reactions that power our world, and the intricate molecular dance that shapes our reality.
Don’t stop here – continue exploring! Dive deeper into specific areas that intrigue you, explore the work of leading scientists in the field, and embrace the wonder of a world defined by atoms and their interactions. The journey of discovery has just begun!





